Biomedical Resource Center
IACUC TRAINING
Purpose: Delineate required training for the IACUC (Institutional Animal Care and Use Committee), investigators who are listed on an IACUC approved protocol and/or personnel involved in the regular animal care or treatment of vertebrate animals and cephalopods covered under a protocol.
Rationale: The Animal Welfare Regulations (AWR) in Section 2.32 require the institution to ensure that all scientists, research technicians, animal technicians, and other personnel involved in animal care, treatment, and use are qualified and trained to perform their duties. Additionally, the institution must make training and instruction available in the specific areas outlined under 2.32(c). Please note: if you are working with biohazardous material or hazardous chemicals, additional training may be required.
Definitions: CITI: Collaborative Institutional Training Initiative, the online training platform which supplies training content
The IACUC requires that certain training must be completed before investigators can work with or provide care to vertebrate animals and cephalopods. This policy also includes personnel involved in regular animal care or treatment of vertebrate animals covered under an IACUC-approved protocol and BRC (Biomedical Resource Center) staff.
IACUC Members
IACUC Members:
Each IACUC member will be provided with a copy of the following:
The PHS (Public Health Service) Policy for the Humane Care and Use of Laboratory Animals.
The National Research Council (NRC): The Guide for the Care and Use of Laboratory Animals.
The ARENA/OLAW IACUC Guidebook.
The AVMA Guidelines on Euthanasia.
A copy of the OLAW (Office of Laboratory Animal Welfare) approved Assurance.
All new IACUC members are provided an orientation that covers the functions of an IACUC as well as an overview of the Animal Care and Use Program which provides training on methods for reporting concerns, humane practices of animal care and use (reduction, refinement, and replacement and methods to minimize pain and distress), use of hazardous agents when working with animals, zoonosis hazards, and what to do in the case of an injury. New members are mentored in protocol review and semi-annual review activities until they are comfortable conducting these activities on their own. In addition, members are required to take the “Essentials for IACUC Members” module offered through www.CITIProgram.org. The Chair and community members are also encouraged to complete CITI courses related to their roles. IACUC members are kept appraised of new policies and procedures via an IACUC dedicated SharePoint site.
Completion of one continuing education course is required annually and ongoing training on assorted topics is provided at a minimum of once a year during a designated IACUC meeting. Continued enrollment in the occupational health program is required.
Surgical Training
When working with rodents in research, maintaining proper aseptic techniques and providing appropriate surgical training are critical for ensuring animal welfare and research validity. The Institutional Animal Care and Use Committee (IACUC) plays a vital role in overseeing and ensuring that the procedures follow ethical guidelines, minimizing pain and distress for the animals.
Surgical Training for Rodents:
Surgical techniques must be learned and practiced to ensure the procedures are performed correctly and efficiently. Proper training and preparation are vital to prevent complications during surgery. All surgeons are required to complete hands on aseptic technique and surgical technique training with the attending veterinarian.
Training Elements:
- Surgical Skill Acquisition:
- Basic Procedures: Trainees often begin with non-invasive techniques and work their way up to more complex surgeries, such as implantation of devices or organ resection.
- Use of Animal Models: Often, training is performed using simulators, cadavers, or other models before conducting live surgeries on rodents.
- Anesthesia and Analgesia:
- Proper anesthesia is essential for rodent surgeries. Training includes understanding the correct dosing of anesthetics (e.g., isoflurane or injectable anesthetics like ketamine/xylazine), monitoring anesthesia depth, and ensuring post-operative analgesia is provided to minimize pain.
- Monitoring and Postoperative Care:
- Trainees should be familiar with how to monitor a rodent’s vital signs (e.g., temperature, respiratory rate, heart rate) during surgery and how to recognize signs of distress or complications.
- Postoperative care should include providing pain relief (e.g., opioids or NSAIDs), monitoring for infection, and ensuring proper recovery conditions.
- Ethics and IACUC Compliance:
- Surgeons must be familiar with the protocols approved by the IACUC, which include ensuring minimal discomfort, appropriate use of anesthetics, and adherence to the 3Rs principle (Replacement, Reduction, Refinement).
- The IACUC ensures that animal welfare standards are met, including appropriate surgical training and proper justification for any procedure.
- Hands-On Experience:
- Adequate hands-on experience under the guidance of an experienced surgeon is critical. Many institutions require trainees to complete a series of supervised surgeries and demonstrate competence before performing unsupervised surgeries.
IACUC Responsibilities in Surgical Training:
- Protocol Review: IACUC reviews all research protocols involving surgery to ensure that proper training, techniques, and post-operative care plans are in place.
- Oversight and Monitoring: IACUC is responsible for monitoring ongoing procedures to ensure that all aseptic practices and ethical standards are followed throughout the study.
- Training Documentation: IACUC may require documentation of training sessions, including certifications of successful completion and competency in the necessary surgical techniques.
Final Considerations:
- Recordkeeping: Maintain detailed records of training sessions, surgical procedures, and post-surgery monitoring. These records should include anesthesia protocols, surgical steps, and any complications that occurred.
- Refinement: Continue to refine surgical techniques over time to minimize harm to the animals. This includes adopting new technologies, improving pain management strategies, and using less invasive methods when possible.
In conclusion, aseptic techniques and proper surgical training for rodents are critical to maintaining high research standards and ensuring the humane treatment of animals. The IACUC’s role in overseeing these practices ensures that animal welfare is upheld while maintaining the integrity of the research.
Prior to training, please review: Principles for the New Surgeon
To schedule a surgicial training, please contact
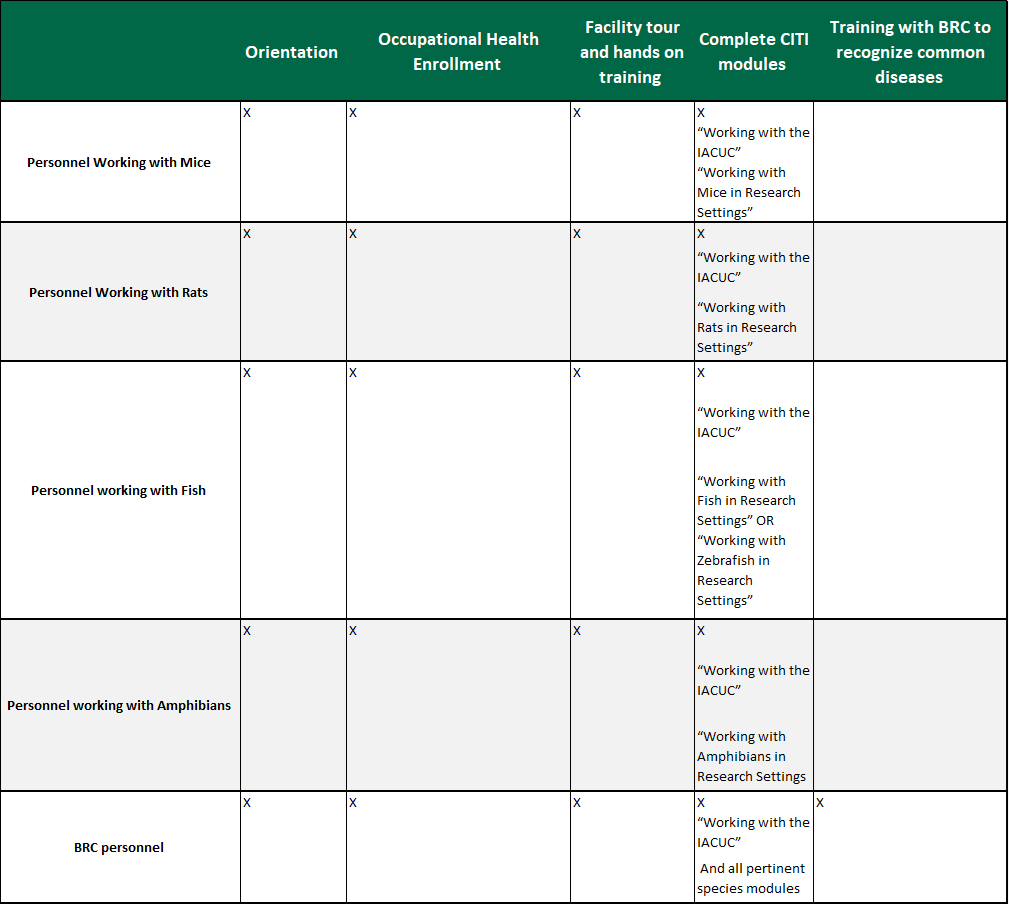
Scientists, Animal Technicians, and other Personnel involved with Animal Care
Scientists, Animal Technicians, and other Personnel involved with Animal Care:
(See Chart Below)
Training must be completed prior to working with animals and prior to protocol approval. All personnel are required to attend an orientation which provides training on methods for reporting concerns, humane practices of animal care and use, reduction, refinement, and replacement, as well as methods to minimize pain and distress, use of hazardous agents when working with animals, zoonosis hazards, and what to do in the case of an injury. A facility orientation is also required during which rodent users receive hands on training for animal handling and restraint. All personnel must be continually enrolled in the occupational health program.
All personnel and supervisors with roles in the care and use of animals at the Institution are to take online CITI training relevant to the species they will be working with as well as the CITI course entitled “Working with the IACUC.” Completion of one continuing education course is required annually. The course must be related to animal welfare or reduction, refinement, and replacement. This can be completed through webinars, CITI courses, AALAS (American Association for Laboratory Animal Science) courses, seminars, conferences, etc. The IACUC has delegated the determination of appropriate applicability of annual continuing education courses to the BRC staff.
BRC personnel must complete the above training and be trained on all BRC standard operating procedures, specific to the responsibilities within the animal care and use program. They are also trained to recognize common laboratory animal ailments.
Personnel performing surgery must complete a training session with the veterinarian on proper use of anesthetics, analgesics, and tranquilizers for the species studied as well as aseptic technique and suture training as applicable to the project.
Training in experimental methods such as specific animal manipulations and techniques will be conducted on an as needed based for the types of research being conducted and the species being studied.
Note: For investigators transferring from other facilities at which they have received similar training, verification of previous training may be accepted in lieu of some Institutional required training. Acceptance of previous training in lieu of the Institution’s training is solely at the IACUC’s discretion.
Zoonotic Agents of Concern
Lymphocytic Choriomeningitis Virus (LCMV)
Testing of purpose-bred mice and murine tumors and cell lines has significantly decreased the potential for transmission of LCMV in the laboratory setting. However, to reduce the potential for transmission, vigilance in screening all murine tissues is required. Tumors may acquire LCMV as an adventitious virus without obvious effects on the tumor. The virus may survive freezing and storage in liquid nitrogen for long periods. Humans become infected by inhaling infectious aerosolized particles of rodent urine, feces, or saliva; by ingesting food contaminated with virus; by contamination of mucous membranes with infected body fluids; or by directly exposing cuts or other open wounds to virus-infected blood. Symptoms are flu-like and include fever, headache, muscle pain and malaise and may progress to more serious illness including meningoencephalitis, lymphadenopathy and neurologic impacts.
Rat-Bite Fever
The risk of rat-bite fever from Streptobacillus moniliformis or Spirillum minor inoculation into the bite wound is minimal due to the eradication of the causative agent from commercial rat colonies. The organisms are found in the respiratory tract and mouths of rats and are typically transmitted via bite wounds. Symptoms develop within 3-10 days, are flu-like and include fever, chills, muscle pain and headache. A rash may develop after fever onset. Without treatment, rat-bite fever can be serious or potentially fatal. Severe illnesses can include infections involving the heart, brain, lungs and abscesses in internal organs.
Campylobacter
Transmission of campylobacter species from animals to humans is through the fecal-oral route. Symptoms develop within two to five days after exposure and include diarrhea, vomiting, abdominal pain, fever, nausea.
Prevention
Wear Protective Clothing: Laboratory coat, disposable gloves.
Hand washing is the most important measure you can take to prevent transmission of zoonotic organisms. Wash your hands with warm water and soap after handling rodents, soiled bedding or soiled cages.
Report injuries or illnesses to your supervisor and Human Resources and/or Campus Police. Cleanse bite and scratch wounds immediately with soap and warm water.
Always tell your treating physician about your research. Regardless of your symptoms, tell your physician about the work you do in the laboratory. Persons with weakened immune systems should seek advice from their practitioner on risks associated with exposure to zoonotic agents in the animal laboratory.
Allergen Statement for Working with Rodents in a Research Setting
Working with rodents in a research setting may expose personnel to allergens such as dander, urine, and saliva, which can lead to allergic reactions or respiratory issues. Symptoms may include sneezing, coughing, skin rashes, and more severe reactions in sensitized individuals. To minimize exposure, personnel are required to use appropriate personal protective equipment (PPE), including gloves, lab coats, and masks, and to follow proper hygiene and cleaning protocols. Those with known allergies to rodents should notify their supervisor, and accommodations or alternative duties may be considered. Regular monitoring and medical support are available to ensure the health and safety of all staff involved in animal research.
